Advanced technology and contemporary processes assure compliance through in-depth validation. PHCbi offers a turnkey, systemized approach to validation and compliance.
We provide turnkey solutions using ISO calibrated instrumentation for validation and qualification in accordance with current GxP regulations (GMP, GLP, GCP), local standards and other regulations.
Request Validation Services
Professional Validation
Our team of experienced factory trained validation service professionals have earned a reputation for professional performance, detailed documentation and outstanding support for our customers. Validation solutions incorporate a data collection and analysis protocol to provide complete and accurate validation reports. All archived data is customer specific with secure backup. We meet international requirements for inspection of pharmaceutical, biotechnology and medical device manufacturing. We use a wired process validation system designed around measurement and reporting requirements of the most intensely regulated industries.
Available Validation Services
On-Site Validation/Qualification
- IOQ (IQ/OQ) Protocol Execution
- Calibration
- Performance Qualification (PQ)
- Validation support
- Consultation
(*Menus for custom validation services provided vary by country. Please contact your local distributor for details*)
Custom Validation
Customized options include but are not limited to:
- Temperature mapping
- Loaded Chamber mapping
- Open Door/Recovery mapping
- Power Failure/Recovery mapping (temperature pull-up)
- Temperature pull-down
- Additional temperature sensor positions
- Extended logging periods – greater than 24 hours
(*Menus for custom validation services provided vary by country. Please contact your local distributor for details*)
Validation Protocols
- Unit specific manufacturer authorized protocol documents
- Traceable to unit model and serial number
- Customizable testing procedures to meet customer specific requirements
- May be purchased separately for in-house or local vendor execution
Validation Solutions
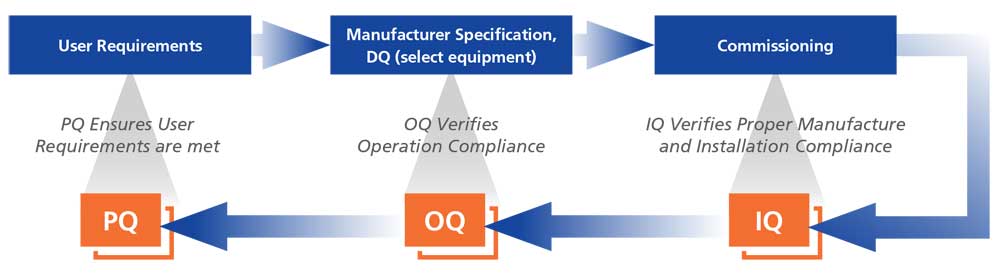
Installation/Operation Qualification (IOQ)
IOQ is performed on the equipment at the customers’ facility. IOQ covers verification of unit conformance to manufacturing specifications including verification of unit assembly and installation (IQ) and verification of unit operation (OQ).
Performance Qualification (PQ)
Performance Qualification challenges the unit by subjecting it to “real world” operational situations for data capture and evaluation. Performance Qualification may include any combination of custom validations like Loaded Chamber Temperature Mapping, Open Door/Recovery Testing and Power Failure/Recovery Testing.
Unique Solutions
Our flexibility to meet your needs and our knowledge of regulatory expectations guide our mission to provide professional and successful completion of our services. Beyond meeting established standards, guidelines and tolerances, we accommodate unique customer application needs. If a service, validation or qualification you require is not listed, please contact us for assistance.
Laboratory Equipment Parameters
Whatever your validation needs, we can provide comprehensive expertise in laboratory equipment to meet your needs.
Laboratory Equipment Parameters
| Product |
Temperature |
CO2 |
O2 |
Humidity
-%RH |
Pressure |
| ULT Freezers
|
|
— |
— |
— |
— |
| Cryogenic Freezers
|
|
— |
— |
— |
— |
| Laboratory Freezers
|
|
— |
— |
— |
— |
| Pharmaceutical Storage Freezers
|
|
— |
— |
— |
— |
| Plasma Storage Freezers
|
|
— |
— |
— |
— |
| Laboratory Refrigerators
|
|
— |
— |
— |
— |
| Blood Bank Refrigerators
|
|
— |
— |
— |
— |
| Pharmaceutical Refrigerators
|
|
— |
— |
— |
— |
| Incubators
|
|
— |
— |
— |
— |
| CO2 Incubators
|
|
|
— |
— |
— |
| CO2/O2 Incubators
|
|
|
|
— |
— |
| Environmental Chambers
|
|
— |
— |
|
— |
Regular scheduling of calibration and preventive maintenance is required to trace performance and ensure the accuracy of your equipment.
Calibration
Validation Services offer compliant calibration to meet your validation and qualification needs. Calibration confirms and verifies that all displayed values and control parameters are within the manufacturer’s tolerances to ensure proper operation at exact set points.
Validation instruments are calibrated to ISO standards and adhere to a regular schedule of calibration and preventive maintenance for ongoing compliance. Test equipment calibration certificates are available on all projects.
Our Professional Expertise
Our validation staff can help you with planning and developing your validation strategies. With our deep knowledge and experience we offer:
- More than 30 years of combined validation experience in the strictest GxP environments
- Field service and service engineering
- 60 years of combined service experience in the laboratory equipment market
- Unbiased testing of competitive equipment
Request Validation Services