Real-time cellular metabolic phenotyping in immuno-oncology research
Central cell metabolism – also known as energy metabolism – describes the interconnected network of biochemical processes that occur within living cells to maintain energy homeostasis and build biomass.1 By studying cell metabolic pathways, researchers can gain functional insights into specific tissue characteristics, develop novel therapeutics and better understand the critical drivers of cell therapy applications.
Metabolic flux is flexible and variable across tissues, cell types and differentiation states.2,3,4
Hence, careful metabolic analyses are essential in supporting basic research and the development of cell-based therapeutics. While all metabolic pathways contribute to cellular function, glycolysis in particular is recognized for its dichotomous role in immune cell health and tumorigenic disease.1,5,6
As it's involved in generating the key intermediates required for cell growth and differentiation, glycolysis often holds a critical role in determining cell fate and function.7,8,9
Typically, glycolysis is responsible for the efficient yet controlled catabolism of glucose into pyruvate, generating adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH) in the process.10,11 Mitochondrial metabolism, notably, oxidative phosphorylation (OXPHOS), is then responsible for meeting the remaining ATP deficit.
In a sufficiently oxygenated environment, glycolysis also yields a small but quantifiable concentration of lactate which is rapidly metabolized by lactate dehydrogenase (LDH). In cancer, aerobic glycolysis is upregulated fueling rapid cell proliferation and increased lactate production.1,6 This phenomenon is broadly termed the Warburg effect and contributes to the unique metabolic profile of tumorigenic cells.
Metabolic intermediates can also function as important intercellular signaling molecules.12,13,14 Therefore, studying the cellular microenvironment also holds relevance in biotherapeutic research. In responsive immune cells, glycolysis drives the effector functions of activated T lymphocytes, yet, their activity may also be suppressed by a lactic acid environment.8,13,14
Live-cell glycolytic analyses can provide functional insights into both the specific tumor microenvironment (TME) and the efficacy of anti-tumordirected cell therapies.15
This article outlines the critical role of live-cell metabolic phenotyping in immuno-oncology research. It also explores the pitfalls of traditional sampling-based methods in contrast to the benefits of continuous monitoring for cell therapy research applications.
Metabolism in immunology and
immuno-oncology research
Metabolism research lies at the core of immune cell therapy development; in fact,
its role in immunology and immuno-oncology research has gained increasing attention in recent years.16,17,18 Immuno-oncology focuses on the application of adaptive immune components as anti-cancer therapies.19 In particular, chimeric antigen receptor (CAR)-T cell technology is a key research focus,having shown great promise both in vitro and in the clinic.
CAR-T cells are designed to express specific receptors on their extracellular surface. These receptors target cancer cell antigens, initiating the recognition and destruction of tumorigenic cells without causing damage to healthy tissue. Metabolic activity is a key indicator of CAR-T cell fitness and function.5,18 In fact, all T lymphocytes undergo significant metabolic reprogramming as they differentiate and mature, shifting from a quiescent to a highly energetic state in support of their effector functions (Figure 1).
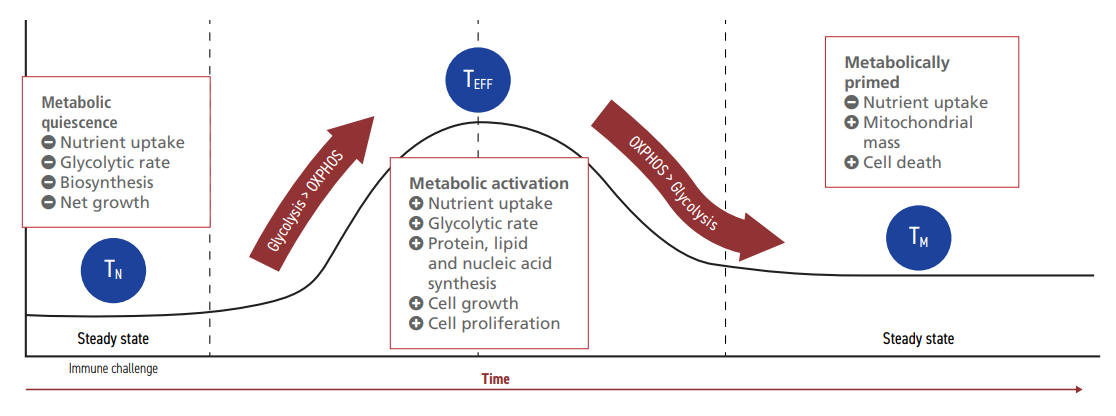
Figure 1: Lymphocyte metabolism during activation and quiescence.20
Naive T cells (TN) circulate in the blood in a steady metabolic state.20 Upon contact with a complementary cancer cell antigen, PI3K-AKT-mTOR signaling pathways are activated, resulting in the expression of transcription
factors that upregulate glycolysis.20,21 If TN can meet the metabolic requirements, they differentiate into effector T cells (TEFF) that can execute immune functions. Once the antigen-presenting cells are cleared, the glycolytic rate of TEFF is reduced. This stimulates a small yet significant number of TEFF to persist as fully differentiated memory T cells (TM). TM exhibit a similar glycolytic profile to TN, although higher rates of mitochondrial respiration enable them to re-activate quickly upon a resurgence of the same threat. If metabolic parameters are not met during
the maturation process, T cells may lose their ability to differentiate or to target tumor cells effectively.22,23,24
Optimizing adoptive cell therapies
The real-time characterization of CAR-T cell metabolic activity can support the understanding of TEFF activation and guide the engineering of optimal therapeutic characteristics.14,21,25,26
These may include increased efficacy and persistence against specific tumor types or effectivity in combination therapy approaches.27,28 For example, CAR-T cells expressing specific costimulatory receptor pairings have been found to possess metabolic characteristics that may render them more effective against acute lymphoblastic leukemia (ALL) than other cell types.27 In addition, certain receptor and coreceptor subdomain pairings can influence the metabolic characteristics of CAR-T cells contributing to a less differentiated TEFF phenotype that has shown to be highperforming in in vivo studies.29
Through a detailed understanding of metabolism,researchers have elucidated the effects of specific transmembrane costimulatory combinations, allowing them to optimize CAR-T cell design for the highest chance of therapeutic success. In addition, the engineered overexpression of specific glycolytic transcription factors can support CAR-T cell persistence in the TME, reducing the impact of lactate-mediated activity suppression.15
Meanwhile, the upregulation of non-glycolytic pathways reduces CAR-T cell exhaustion and increases their therapeutic persistence.30
Without accurate metabolic analyses, all such research efforts could be limited in their applications.
Immuno-oncology therapeutics are often administered as part of a combination therapy or multi-drug approach.31,32 Combination therapies are an exciting area of research surrounding the treatment of solid tumors.33,34 Programmed Cell Death Protein 1 (PD-1) is an immune response inhibitor that has been widely targeted in anti-tumor therapies.35 PD-1 exists on the surface of many immune cell types; it can interact with its complementary ligand on the tumor cell surface and offer a mechanism for tumor immune escape.28,35 PD-1 stimulation can also abrogate CAR-T cell glycolytic rates and result in a metabolic shift toward mitochondrial respiration.35
Hence, metabolic analyses can guide CAR-T cell engineering and, in the face of potential immune escape, support the design of therapeutic persistence alongside small molecule PD-1 inhibitors.
Challenges and opportunities in cell glycolytic phenotyping
Several metabolic phenotyping approaches currently exist to support immuno-oncology research. The latest technologies utilize probe-based methods to measure key metabolic parameters in cell-adjacent media (oxygen consumption, extracellular acidification or changes in metabolite concentrations). While such methods claim to provide dynamic, real-time insights into cellular energy homeostasis, traditional sampling-based methods can fail to capture critical glycolytic data.
Figure 2: The latest live-cell metabolic phenotyping technology for continuous sampling of glucose and lactate production.38
Lactate production rate is one parameter by which
researchers can measure glycolytic activity (Figure 2).36 It
can also be used to evaluate changes in the balance of
other metabolic processes. However, the rapid clearance
rate of this critical metabolite means that samplingbased methods can easily underestimate glycolytic
function.37 Additionally, the highly flexible nature of
CAR-T cell metabolism means rapid changes can easily be missed.4,5
Continuous measurements, taken without
undue cell or media disturbance, can therefore offer
superior accuracy to their sampling-based counterparts.
By measuring glucose consumption and lactate
production, researchers can take a more direct approach
to measuring metabolic flux with a direct inference of
ATP production. Hence, continuous measurements and
considered experimental design can enable researchers
to easily and clearly analyze TEFF activation without
the risk of data misinterpretation from anomalous or
inaccurate results.

While the advent of instruments for live-cell metabolic
analysis has significantly enhanced the scope for in
vitro research, many methods include complex cell
culture requirements such as specialist media and/
or re-plating and de-gassing protocols. Not only can
such requirements be costly in time and resources,
but they may also induce unwanted treatment effects
and confound important controls. Therefore, analyzer
protocols that take real-time measurements from within
standard culture vessels without undue processing can
be considered a more robust experimental approach.
Additionally, they can support research on hard-toculture or non-adherent cell populations while boasting
affordability and compatibility with existing lab systems and infrastructure.
The latest technology in continuous live-cell metabolic
phenotyping is capable of offering real-time observations
of cultured cells in the standard environment (Figure
2). Thus, it offers greater consistency, flexibility and
reproducibility than other methods, and can provide
data that is directly comparable to other supporting
experiments.
It is essential that real-time metabolism data is both
accurate and robust if technological advancements
are to be made. Continuous monitoring not only
supports the exclusion of anomalous results, but it also
provides unrivaled insights into CAR-T cell metabolic
reprogramming.38
During experiments, it may be
pertinent to measure the metabolic effects of coreceptor
engineering over time; thus uncovering differences in cell
differentiation or persistence as they occur.15,31,39,40 This helps researchers select appropriate cell populations for
further analysis or clinical applications. With continuous
monitoring, it is possible for researchers to observe realtime metabolic changes as they occur, providing the
best understanding of how novel therapy designs may
differentiate and mature in vitro.38,40
Conclusion and future perspectives
Real-time metabolic phenotyping is a crucial aspect of
immuno-oncology research and can pave the way for the
development of more advanced adaptive cell therapies.
Understanding the metabolic dynamics of immune cells is
vital for optimizing their efficacy. Meanwhile, metabolic
analyses can also provide insights into the TME and the
potential challenges it poses for in vivo applications.
Metabolic phenotyping could be used to provide
insights into the activation and function of CAR-T cells,
guiding their engineering and optimizing therapeutic
outcomes. However, the future of such technologies will
likely focus on the application of continuous monitoring
approaches to increase the reliability, robustness and
publication potential of cell metabolic data. For this
reason, continuous monitoring is likely to become the
gold standard approach in metabolic research with
significant impacts on the field of immuno-oncology.
References
- Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34(3):355-377.
doi:10.1016/j.cmet.2022.01.007
- Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood:evaluation by mechanistic model of resting energy
expenditure. Am J Clin Nutr. 2010;92(6):1369-1377.
doi:10.3945/ajcn.2010.29885
- Gebert N, Rahman S, Lewis CA, et al. Identifying celltype-specific metabolic signatures using transcriptome and proteome analyses. Curr Protoc. 2021;1(9):e245.
doi:10.1002/cpz1.245
- Peng M, Li MO. Metabolism along the life journey of T cells. Life Metab. 2023;2(1):load002.
doi:10.1093/lifemeta/load002
- DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21(12):785-797.
doi:10.1038/s41577-021-00541-y
- de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol. 2019;9:1143.
doi:10.3389/fonc.2019.01143
- Ediriweera MK, Jayasena S. The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites. 2023;13(3):345.
doi:10.3390/metabo13030345
- Hortová-Kohoutková M, Lázničková P, Frič J. How immunecell fate and function are determined by metabolic pathway choice: The bioenergetics underlying the immune response. Bioessays. 2021;43(2):e2000067.
doi:10.1002/bies.202000067
- Chi H. Immunometabolism at the intersection of metabolic signaling, cell fate, and systems immunology. Cell Mol Immunol. 2022;19(3):299-302.
doi:10.1038/s41423-022-00840-x
- Naifeh J, Dimri M, Varacallo M. Biochemistry, Aerobic Glycolysis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK470170/. Accessed November 01, 2023.
- Jones W, Bianchi K. Aerobic glycolysis: beyond proliferation. Front Immunol. 2015;6:227.
doi:10.3389/fimmu.2015.00227
- Haas R, Cucchi D, Smith J, Pucino V, Macdougall CE, Mauro C. Intermediates of Metabolism: From Bystanders to Signalling Molecules. Trends Biochem Sci. 2016;41(5):460-471.
doi:10.1016/j.tibs.2016.02.003
- Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812-3819.
doi:10.1182/blood-2006-07-035972
- Rostamian H, Khakpoor-Koosheh M, Jafarzadeh L, et al. Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer. 2022;22(1):39.
doi:10.1186/s12885-021-09151-2
- Mangal JL, Handlos JL, Esrafili A, et al. Engineering Metabolism of Chimeric Antigen Receptor (CAR) Cells for Developing Efficient Immunotherapies. Cancers (Basel).2021;13(5):1123.
doi:10.3390/cancers13051123
- Pajai S, John JE, Tripathi SC. Targeting immune-oncometabolism for precision cancer therapy. Front Oncol. 2023;13:1124715.
doi:10.3389/fonc.2023.1124715
- Bader JE, Voss K, Rathmell JC. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol Cell. 2020;78(6):1019-1033.
doi:10.1016/j.molcel.2020.05.034
- Leone RD, Powell JD. Fueling the Revolution: Targeting Metabolism to Enhance Immunotherapy. Cancer Immunol Res. 2021;9(3):255-260.
doi:10.1158/2326-6066.CIR-20-0791
- Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: Current progress and prospects. Front Immunol. 2022;13:961805.
doi:10.3389/fimmu.2022.961805
- Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454.
doi:10.1126/science.1242454
- Zhang M, Jin X, Sun R, et al. Optimization of metabolism to improve efficacy during CAR-T cell manufacturing. J Transl Med. 2021;19(1):499.
doi:10.1186/s12967-021-03165-x
- Raynor JL, Chapman NM, Chi H. Metabolic Control of Memory T-Cell Generation and Stemness. Cold Spring Harb Perspect Biol. 2021;13(6):a037770.
doi:10.1101/cshperspect.a037770
- Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2(10):1001-1012.
doi:10.1038/s42255-020-00280-9
- Sugiura A, Rathmell JC. Metabolic Barriers to T Cell Function in Tumors. J Immunol. 2018;200(2):400-407.
doi:10.4049/jimmunol.1701041
- Rangel Rivera GO, Knochelmann HM, Dwyer CJ, et al. Fundamentals of T Cell Metabolism and Strategies to Enhance Cancer Immunotherapy. Front Immunol. 2021;12:645242.
doi:10.3389/fimmu.2021.645242
- Luby A, Alves-Guerra MC. Targeting Metabolism to Control Immune Responses in Cancer and Improve Checkpoint Blockade Immunotherapy. Cancers (Basel). 2021;13(23):5912.
doi:10.3390/cancers13235912
- Kawalekar OU, O’Connor RS, Fraietta JA, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells [published correction appears in Immunity. 2016 Mar 15;44(3):712]. Immunity. 2016;44(2):380-390.
doi:10.1016/j. immuni.2016.01.021
- Zheng JB, Wong CW, Liu J, et al. Glucose metabolism inhibitor PFK-015 combined with immune checkpoint inhibitor is an effective treatment regimen in cancer. Oncoimmunology. 2022;11(1):2079182.
doi:10.1080/2162402X.2022.2079182
- Rial Saborido J, Völkl S, Aigner M, Mackensen A, Mougiakakos D. Role of CAR T Cell Metabolism for Therapeutic Efficacy. Cancers (Basel). 2022;14(21):5442.
doi:10.3390/cancers14215442
- Li W, Zhang L. Rewiring Mitochondrial Metabolism for CD8+ T Cell Memory Formation and Effective Cancer Immunotherapy. Front Immunol. 2020;11:1834.
doi:10.3389/fimmu.2020.01834
- Xiao X, Wang Y, Zou Z, et al. Combination strategies to optimize the efficacy of chimeric antigen receptor T cell therapy in haematological malignancies. Front Immunol. 2022;13:954235.
doi:10.3389/fimmu.2022.954235
- Wang S, Sellner L, Wang L, et al. Combining selective inhibitors of nuclear export (SINEs) with chimeric antigen receptor (CAR) T cells for CD19positive malignancies. Oncol Rep. 2021;46(2):170.
doi:10.3892/or.2021.8121
- Ramello MC, Haura EB, Abate-Daga D. CAR-T cells and combination therapies: What’s next in the immunotherapy revolution?. Pharmacol Res. 2018;129:194-203.
doi:10.1016/j.phrs.2017.11.035
- Wang AX, Ong XJ, D’Souza C, Neeson PJ, Zhu JJ. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front Immunol. 2023;14:1140541.
doi:10.3389/fimmu.2023.1140541
- Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front Pharmacol. 2021;12:731798.
doi:10.3389/fphar.2021.731798
- Harrison D, Wu D, Huang J, Fang Y. Single-cell lactate production rate as a measure of glycolysis in endothelial cells. STAR Protoc. 2021;2(3):100807.
doi:10.1016/j. xpro.2021.100807
- Li X, Yang Y, Zhang B, et al. Lactate metabolism in human health and disease [published correction appears in Signal Transduct Target Ther. 2022 Oct 31;7(1):372]. Signal Transduct Target Ther. 2022;7(1):305.
doi:10.1038/s41392-022-01151-3
- In-Line monitoring of culture medium using Live cell metabolic analyzer. PHC Corporation. https://pages.services/markitbiomedical.com/live-cell-in-line/. Accessed November 02, 2023.
- Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692.
doi:10.1038/ncomms7692
- Metabolic rate monitoring of undifferentiated iPS cells and expression analysis of differentiation markers. PHC Corporation. https://www.phchd.com/jp/-/media/biomedical/asia-pacific/jp/landing_page/live-cell/LiveCell_WP03_en.pdf
Accessed December 07, 2023.