We have been contributing to the development of healthcare as a leading provider of medical devices and in vitro diagnostics (IVD) for many years, developing Japan's first clinical diagnostic reagent in 1962 and the industry's first blood glucose monitoring system in 1991.
Since our carve-out from the Panasonic Group in 2014, we have continued to develop high-precision, high-reliability, and high-value-added products through our outstanding development and manufacturing technologies, and provide products based on customer feedback (opinions and requests).
We also support early detection and effective treatment of various diseases together with our collaborative partners by providing high-performance medical equipment.
We are committed to delivering products and services that contribute to the realization of better medical care by making full use of the technological, manufacturing, and quality capabilities we have cultivated over the years.
Strengths
- VOC(Voice of Customer)
- Listening to our customers to better understand their needs Front-loading concurrent engineering (Quality departments are involved from the planning and design stage)
- Optimized production processes
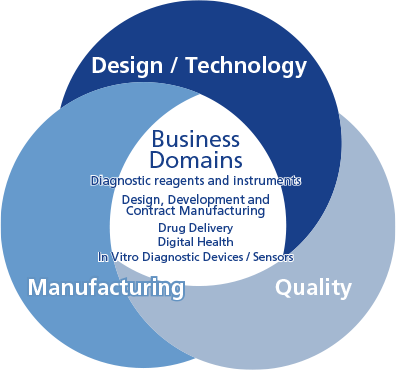

- Planning
- Industrial Design
- Sensors/Reagents
- Electric Circuits
- Hardware/Software
- Packaging
- Prototype/Performance Evaluation
- Technical Standards

- Product Certification
- Manufacturing Technology
- QMS/GMP certification

- Quality Control
- Quality Assurance
- Traceability