
Many laboratories and clinical facilities need cleanrooms for a variety of reasons. Contaminants or particles in the air greatly impact the process of both testing and manufacturing samples and products. The creation of particles by certain laboratory. equipment can lead to accelerated degradation, contamination and total loss of biologically relevant material.
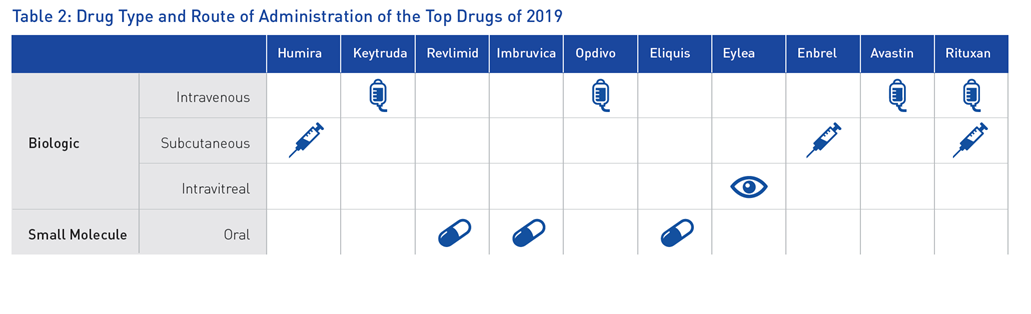
Out of the top 10 best-selling drugs of 2019, 7 were biologics (Table 2).11 When manufacturing biological products, it is imperative to acknowledge that any change during the manufacturing process, regardless of how minor, may alter product quality and efficacy.12 Therefore, within GMP facilities, it is important to classify your clean areas accurately. One of the first steps in this process is implementing cleanroom certified furniture and equipment.
GMP compliance can be achieved when consistent GMP-grade materials from well-characterized sources are implemented and utilized.
What is a Cleanroom Classification?
A cleanroom is a controlled environment where the concentration of airborne particles like dust, microbes and aerosol particles are controlled. Cleanrooms are maintained and utilized in a manner that minimizes the introduction, generation and retention of particles in the environment. All cleanrooms that meet the GMPs are classified according to the cleanliness level of the air inside them.
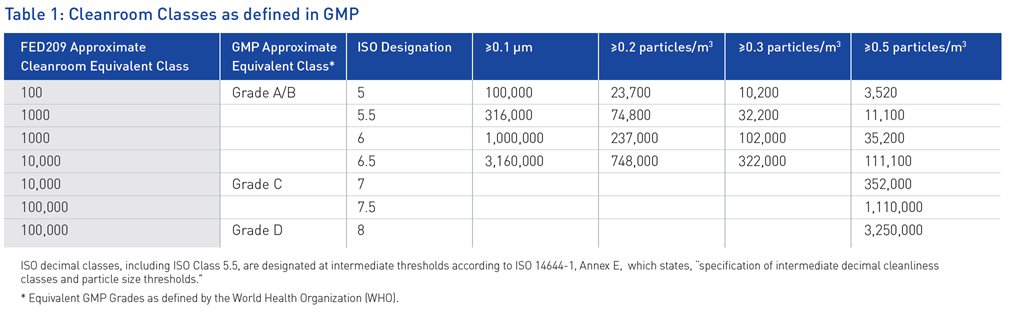
Meeting requirements for cleanrooms is one of the most critical issues for laboratories adhering to GMP requirements. The International Organization for Standardization (ISO), a nongovernmental body charged with promoting worldwide standards to ensure safe, reliable and high-quality products, developed classifications associated with the levels of cleanroom certification. ISO awards cleanroom designations based on a threshold of allowable particles within a specified area (Table 1). According GMPs requirements, cleanroom classifications should be carried out according to ISO 14644-1. This ISO classification impacts every cleanroom user in the GMP community.
All preservation products with Cleanroom Classification